-
Discovering Group Theory @Hampshire, Days 24-27
For the last two weeks of the semester students worked independently on projects which involved the material we learned in class.The class time was spent on research and discussion of their individual projects.
A first-year art student explored and identified the approximate symmetries of geometric shapes and symbols of ancient South-Western petroglyphs and rock paintings, which included dihedral groups and the translation group. A third-year student with a concentration in math researched the application of group theory to Rubik’s cube.(Photo by By Lars Karlsson (Keqs), Own work, via Wikimedia Commons) A third-year art student researched the connections between group theory and the tree branching patterns. A first-year student, still undecided on her concentration, dove into a paper on applications of group theory to molecular systems biology and used what we learned in class to understand the key points made in the article (Rietman EA, Karp RL, Tuszynski JA. Review and application of group theory to molecular systems biology. Theoretical Biology & Medical Modelling. 2011;8:21. doi:10.1186/1742-4682-8-21.) A second-year pre-med/math student researched the use of group theory in chemistry. - A second-year pre-med/math student researched the use of group theory in chemistry.
- A first-year student, still undecided on her concentration, dove into a paper on applications of group theory to molecular systems biology and used what we learned in class to understand the key points made in the article (Rietman EA, Karp RL, Tuszynski JA. Review and application of group theory to molecular systems biology. Theoretical Biology & Medical Modelling. 2011;8:21. doi:10.1186/1742-4682-8-21.)
- A first-year art student explored and identified the approximate symmetries of geometric shapes and symbols of ancient South-Western petroglyphs and rock paintings, which included dihedral groups and the translation group.
- A third-year art student researched the connections between group theory and the tree branching patterns.
- A third-year student with a concentration in math researched the application of group theory to Rubik’s cube.
The course concluded with class presentations.
After I complete narrative evaluations for the students in the course, I will write a post reflecting on my own experience teaching this course.
-
Discovering Group Theory, Day 20
On Day 20, after reviewing the Galilean group and the classic formula for the addition of velocities, we went on to talk about their incompatibility with the results of the Michelson–Morley experiment. We begun to resolve the issue by deriving the expression for time dilation using the example of a moving light clock.
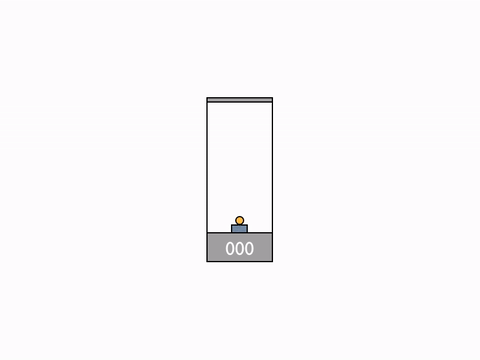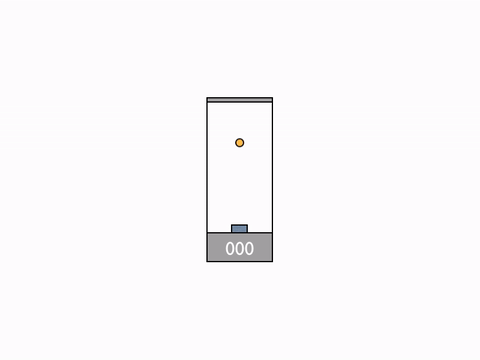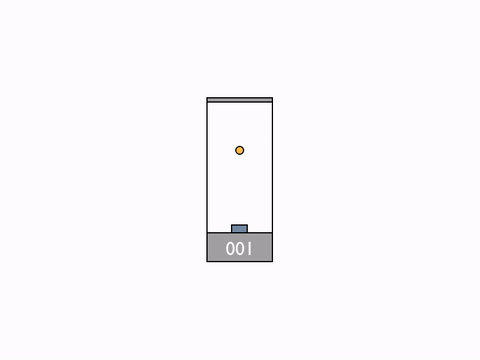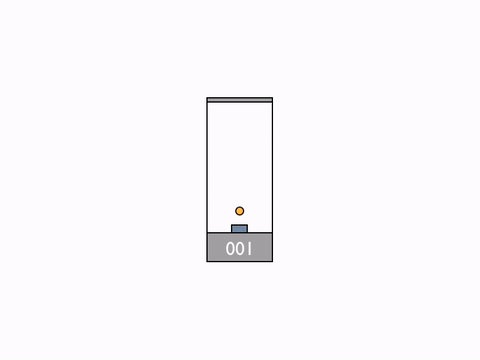
Light clock -
Color Physics @Hampshire, Day 21
On Day 21, we first reviewed the interaction of light and matter. Students then used hand-held spectrometers to observe and record the spectrum of hydrogen and identified the mystery gas in one of the tubes.

Students used hand-held spectrometers to observe and record spectra of two gases. -
Color Physics @Hampshire, Day 20
On Day 20, we covered two different topics.
We first discussed how reflection and refraction affect the saturation of pigments.
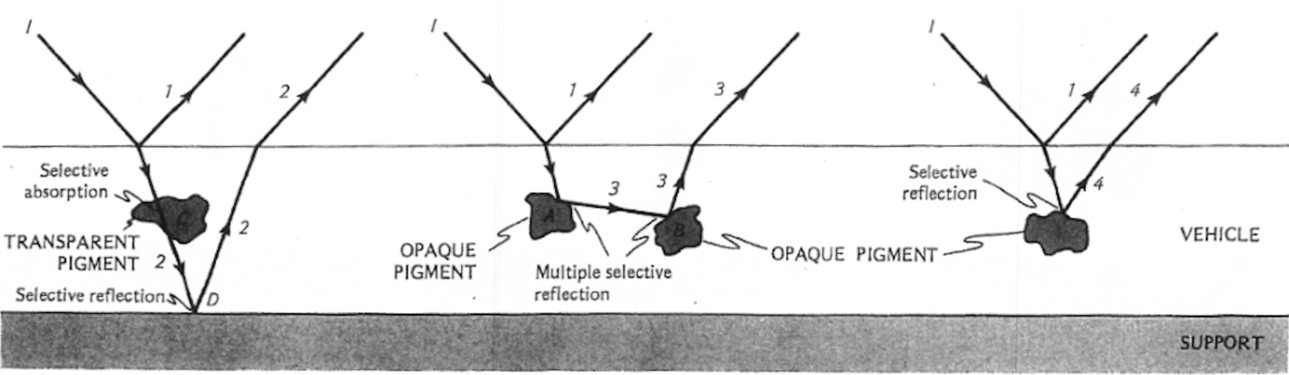
Reflection and Refraction in pigments from Seeing the Light: Optics in Nature, Photography, Color, Vision, and Holography by David R. Falk, Dieter R. Brill , David G. Stork. Wiley, 1986.
We then moved on to some basic quantum mechanics in order to explain why various pigments absorb and reflect different wavelengths. We used a simple model of atom energy levels of sodium. New concepts introduced included
- Photons and their energy E=hc/λ
- Quantized atomic energy levels
- Energy levels of sodium
- Emission and absorption spectra of gases
Sources: Nassau, Kurt “The Causes of Color,” Scientific American, October 1980, pages 124-154.
-
Color Physics @Hampshire, Day 19
On Day 19 we studied reflection and refraction. Students used light sticks, mirrors, and acrylic blocks to investigate, both qualitatively and quantitatively, the optical behavior of light at the interface of material with different indices of refraction. Their investigations led us to the law of reflection and Snell’s law.*
We introduced a number of new concepts:
- index of refraction
- normal to the surface
- angle of incidence
- angle of reflection
- angle of refraction/transmitted angle
Students sent red LED light through acrylic blocks and investigated its reflection and refraction angles. Students sent red LED light through acrylic blocks and investigated its reflection and refraction angles. *The photgraphs taken by and used with the permission of Andrew Hart of Hampshire College.
-
Color Physics @Hampshire, Day 18
On Day 18 we
- Discussed the pieces students drew/painted over the course of the last three days
 A halftone exercise with watercolors by Madeleine Perreault.
A halftone exercise with watercolors by Madeleine Perreault.
The effects of additive mixing. Piece by Sara Young, looks grey from far away, but up close it is a colorful mixture. - Practiced how to determine the spectral composition of reflected light from the spectral composition of incident light and the reflectance curves of the surfaces.
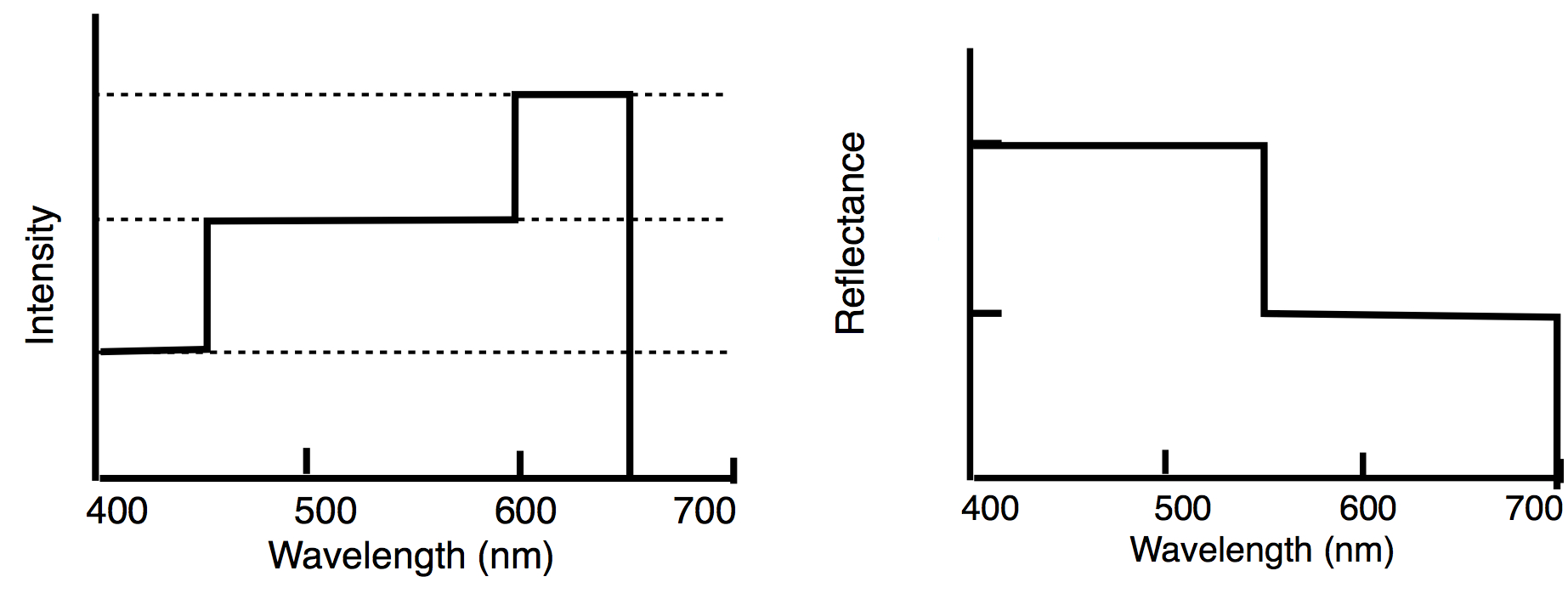
Given date for a sample problem for which students used the incident intensity distribution and the reflectance curve to find the intensity distribution of the reflected light. -
Discovering Group Theory, Day 17
Realizing that space-time symmetries provide more intuitive introduction to the abstract notion of a symmetry used in physics, on day 17 we switched gears by discussing Richard Feynman’s lecture “Symmetry in Physical Law,” which students watched outside the class.
Feynman introduces the notion of a symmetry of a physical law, which we disected in the context of Coulomb’s law.
- We defined a coordinate system and discussed the difference between passive and active transformations.
- We worked out the translational and rotational spatial symmetries in 2D and showed that translations form a group.
- We also introduced translations in time and showed how they leave the kinematic equations unchanged.
As a transition to the discussion of moving observers, we watched the first half of a wonderful video from the 60’s titled Frames of Reference.
-
Discovering Group Theory, Day 16
On Day 16, we finished up complex numbers in polar form and introduced several new concepts:
- The circle group T and unitary group U(1)
- Infinite vs finite groups
- Continuous vs discrete groups
We also started going through t’Hooft’s Scientific American article “Gauge Theories of the Forces between Elementary Particles“, which proved to be challenging but exciting. We discussed the Standard Model of particle physics and the distinction between spacetime symmetries and internal symmetries.
-
Color Physics @Hampshire, Days 15-17
Days 15, 16, and 17 were spent on hands-on activities in which students investigated subtractive mixing by reflection, and explored the use of additive and subtractive mixing in print and painting. The activities included:
- Creating partitive mixtures in the pointillist style

- Investigating and replicating the use of halftone in print


Students used magnifying glasses to look at newsprint and determined how the CMYK system is used for printing. Then the made their own halftone designs.
- Investigating subtractive mixing by reflection.
 This portion involved three different activities:
This portion involved three different activities:
- In the first, students placed pieces of paper of various colors in a light-tight box, illuminated them by one of the lights we have (red, green, or blue), and recorded their observations. The papers we used were to an OK approximation, red, blue, green, cyan, magenta, and yellow.
- In the second activity, students used their observations from the first to predict what colors the same pieces of paper would appear to have when illuminated by white light, but viewed through filters of different colors. They then tested their predictions and commented on any discrepancies.
- Finally, using what they learned from the first two activities, students devised a method by which they can determine how “pure” the cyan, magenta, and yellow pigments in their watercolor sets were.
- Holography. Thanks to Prof. Wirth, each student got to make a hologram using reflection holography.
made holograms today in my physics of color class ? @physartphil pic.twitter.com/9Hu4I7clzY
— sara (@saraannyong) April 6, 2017

A student-made hologram of a thread spool
- Creating partitive mixtures in the pointillist style
-
Discovering Group Theory, Day 15
On Day 15, we continued working with complex numbers, but now in exponential or polar form. After an introduction to complex numbers exponential form and its relation to the rectangular form, students worked on exercises covering:
- Complex numbers in the polar form and their relation to the rectangular form
- Complex conjugation and modulus
- Complex numbers as points in a complex plane
About half of the class managed to get to the proof that the complex numbers form a group under multiplication, which was easier to do in the polar form.
We ended the class by watching a TED talk by Murray Gell-Mann, “Beauty, truth, and …, physics?”.






